Microbial Ecology of the Built Environment
Urbanization and our increasingly indoor lifestyle have deeply affected how we acquire and interact with our microbiota. At the Gilbert Lab, we are working to answer fundamental questions about our microbial interaction with built environments, including what factors influence their microbial communities and how microbes are transferred throughout these environments. Much of our research relies on longitudinal surveys, including our Home Microbiome Project, which recruited citizen scientists to collect samples of their home and skin microbiota over time, as well as our Hospital Microbiome Project, which collected samples from patients, hospital staff, and surfaces of a newly constructed hospital over the course of its first year. Moreover, humans aren’t the only species who inhabit built environments. We are working with the Shedd Aquarium in Chicago to characterize how the different microbial communities throughout the aquarium are intertwined day-to-day and how they are affected by their surrounding built environment. For instance, we want to know what microorganisms in the water interact with the dolphin’s own microbiome, and which ones could prime the animal’s immune system to make them stronger against invading pathogens.
Environmental Microbial Ecology & Biogeochemistry
Microbiological water quality has broad implications for economic, health, and environmental fluctuations. It is often complicated to monitor pathogens directly as a means to assessing water quality due to their low abundance in natural river systems; in addition, they are often difficult to culture and have intermittent distributions. In conjunction with scientists at Argonne National Laboratory and the Metropolitan Wastewater Reclamation District of Greater Chicago (MWRD), we are striving to develop high-throughput 16S rRNA- and metagenomic-based assays for the assessment of ecosystem health in the Chicago River. Additionally, biogeochemical cycling is crucial to ecosystem functioning in order to maintain a balanced influx and efflux of nutrients. Microorganisms are pivotal in nutrient transformation processes, therefore changes in microbial communities caused by pollutions or anthropological stresses may disrupt the whole ecosystem, including humans. For instance, we are monitoring the impact of the increase in greenhouse gas emissions on microbiomes in urban soil to assess the change in diversity and structure of the overall microbial community as well as the functional groups involved in biogeochemical cycling.
The Human Microbiome
Research has suggested that bacterial probiotic therapy may prevent and reduce the symptoms of depression, possibly through both immune stimulation and the production of neuro-active compounds. Therefore, manipulating the microbial assemblages in the gut could represent a novel therapeutic for perinatal depression. The gut-brain axis mediates the interaction between bacterial communities in the intestine and neurological, immunological and endocrinological processes. Microbial immune activation and neurotransmitter metabolism (e.g. dopamine, 5-Hydroxytryptophan (5-HT), a precursor for serotonin, γ-Aminobutyric acid (GABA), noradrenalin, and acetylcholine) can directly and indirectly influence brain function, especially through the hypothalamic-pituitary-adrenal (HPA) axis. The HPA axis releases corticosteroids (i.e., cortisol) and activates downstream neurons that secrete neurotransmitters (i.e., norepinephrine), which can then modulate the gut ecosystem via immune-mediated antimicrobial peptides. Importantly, many of these interactions can be inhibited by severing the vagus nerve, thus cutting the main communication link between brain and gut.
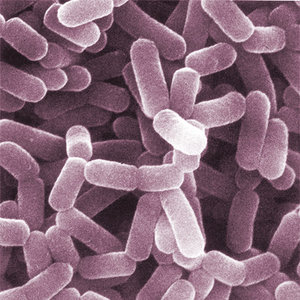
Medical Implications
The human microbiota consists of 100 trillion microorganisms and vastly outnumbers the human genetic repertoire. These microorganisms have been shown to affect a number of processes, including training the immune system to respond to infections and affecting reproductive health outcomes such as preterm births and post-partem depression. We continue to explore the intricate relationship between our microbiota and our health through our medical research with prominent universities throughout Chicago.
Wildlife-Microbe Interactions
Diversity of microbial symbionts in wild animals is a growing area of interest. Studies of wild animal and their symbionts can yield insights into the eco-evolutionary dynamics between hosts and symbionts, provide baseline information for the health of wild populations, and address important questions linking symbionts to viral pathogenesis and transmission. The Gilbert Lab is addressing these topics in a number of wild host-symbiont systems.
The Bat Microbiome Project: Bats are the second-most diverse group of mammals on the planet, and exhbit a broad range of ecological and evolutionary characteristics. Led by Dr. Holly Lutz, this project explores the variation of microbial symbionts in bats, the eco-evolutionary biology of these host-symboint relationships, and links between microbes and blood-borne pathogens. House Sparrow (Passer domesticus)
Microbiome Project: This project, headed by graduate student Sophia Carryl, aims to understand how differences in microbiome impact house sparrow fitness. Microbial influences on fitness can reveal a key proximate mechanism that shapes the evolution of house sparrows’ mating, parental and social behaviors. These influences can also help address the recent decline in house sparrow populations in response to an increasingly urban world.
Biodiversity and microbial inventories in African wildlife: In conjunction with the Field Museum of Natural History, Dr. Holly Lutz is leading a broad-scale effort to assess the diversity and function of microbial symbionts in African vertebrates. Afrotropical ecosystems are under increasing pressure from anthropogenic disturbance, and with the establishment of baseline data for communities in these systems, microbes may serve as important signatures of health in wild populations..